ABOUT DESITIN
- Home
- /
- Research & Development
Research & Development
At Desitin, we are proud that for over 100 years we have consistently produced global innovations that have considerably improved and simplified drug treatment for doctors and patients. Many of these innovations have already become standard treatments in many countries.
In bringing innovations to the market, we always will work
closely leading experts and treatment centres. It is due to our close collaboration that we have built strong relationships and reputation with health professionals, pharmacists, patients and other businesses in the pharmaceutical industry, as experts in the field of CNS medicines.
Desitin – Our distinguished history in the treatment of epilepsy.
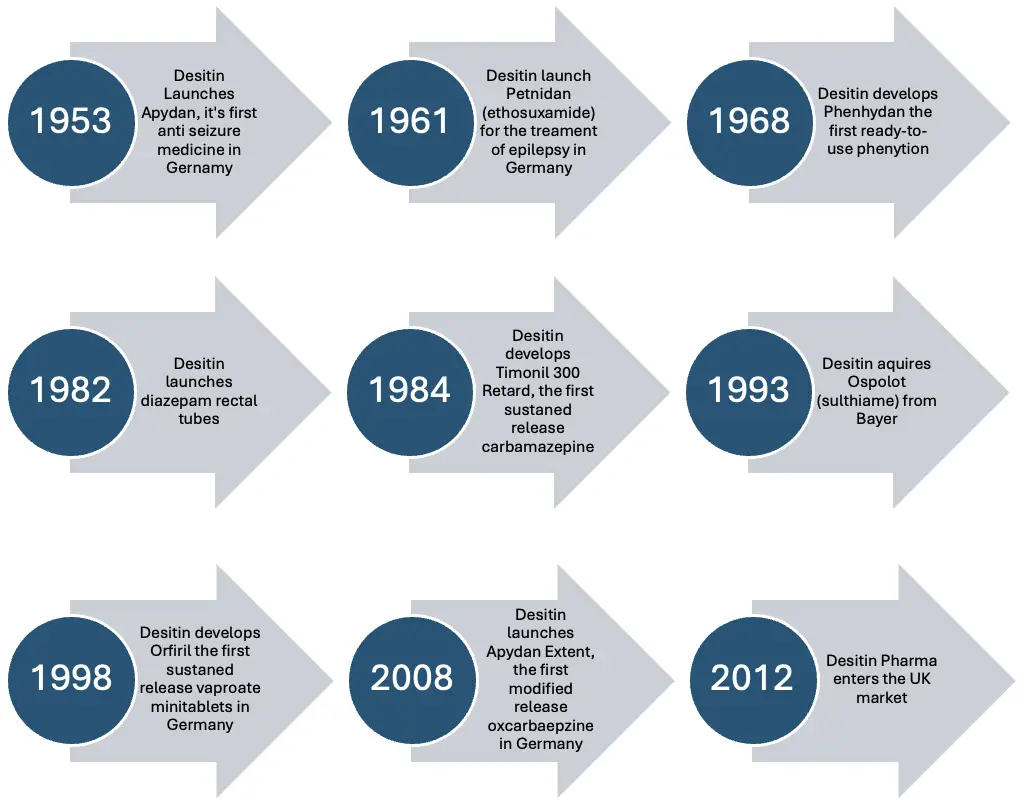
Please note that not all of the above products are marketed in the UK

